This article is part of the network’s archive of useful research information. This article is closed to new comments due to inactivity. We welcome new content which can be done by submitting an article for review or take part in discussions in an open topic or submit a blog post to take your discussions online.
What is it?
The TDR Global Competency Framework for Clinical Research is a flexible framework which lists all the competencies that should be demonstrated by a research team to carry out a successful clinical study. The framework can be applied to any clinical research study, regardless of the size of the team, place, disease focus and type of research. Together with its supporting tools, the framework can be used to plan staffing requirements for a study, to carry out appraisals of staff, to guide career development, and to create educational curricula for research staff.
The Special Programme for Research and Training in Tropical Diseases (TDR) and researchers from The Global Health Network led the development of this framework, to bridge the lack of information about clinical research roles, especially for those working in low and middle income countries (LMICs). It has been developed alongside experts and collaborators from around the world.
What are the parts of the framework, and how does it work?
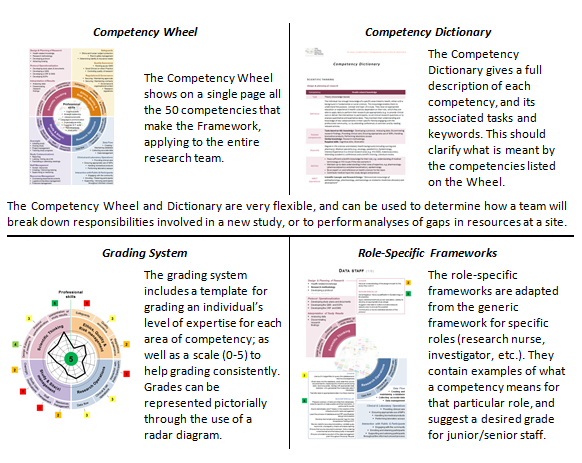
The framework consists of several interlinked parts which can be used together. You can download and use them from the 'Useful Resources' section on the right hand side of this screen.
(Please note that the role specific frameworks are under construction)
While the Competency Wheel and Dictionary form the core of the framework, the Grading System and Role-Specific Frameworks (in prepartaion) can help while developing job descriptions, appraising staff, creating training curricula, etc.
On the right hand side of the screen ('Useful Resources' section), you will find all the above documents, but also:
- A report explaining the methodology for the development of the framework
- The protocol and feedback forms for using and pilot-testing the framework
If you use the framework, please make sure that you complete the feedback form. It only takes a few minutes, and will help us to improve the framework for future users.
Why has this been developed? Many frameworks already exist.
Many high quality frameworks for roles within clinical research do indeed already exist, but they have several limitations that we tried to address by adopting a wide scope to development:
- Frameworks were usually developed for one single job role, and/or one location. However, teams vary considerably depending on the study, its location, and the size of the team. For example, a research nurse may specifically be involved in informed consent, sample taking and clinical care of study participants; while in another setting and smaller trials, they may commonly also take on trial coordination tasks. Therefore it’s interesting and useful to consider job roles together as a continuum rather than solely in isolation.
- Frameworks have been developed in different ways for different roles; and mostly relying on experts’ opinion. Combining those viewpoints could help to standardise the information, and the resulting picture should be enriched with real-life examples of the tasks and competencies required for each role. There are also few frameworks which have been developed taking into account Low and Middle Income Countries (LMICs).
- Most frameworks have been designed to highlight competencies needed to run clinical trials , especially as performed to assess drug efficacy. This approach has some value, especially for when aiming at market approval of a drug, but it neglects other important types of clinical research such as observational studies. It is important to bridge the gap between the two, so that similarly high standards are applied to all, while researchers know when or not to abide by stringent regulations depending on the aim of their study.
How has this been developed?
In agreement with TDR, researchers from The Global Health Network identified the roles to be covered; and performed a literature review to locate existing high-quality frameworks and documents outlining the responsibilities for certain clinical research roles. The information contained on the competencies and tasks required during a study was systematically categorised into themes. Initially, a few broad themes (inspired from the most generic pre-existing framework, developed by the The Multi-Regional Clinical Trials Center (MRCT) of Brigham and Women's Hospital and Harvard) were used to categorise the text data.
Job descriptions were then collected from global partners and from the internet, to cover a broad range of clinical study types and locations (including LMICs). Those were analysed as described above, which led to the creation of new, more specific themes to cover all aspects of the rich data collected. The process continued until 116 job descriptions were included, alongside the 28 frameworks and guidelines. At this point, the analysts were satisfied no new theme was needed to describe the data.
All data was then re-examined for consistency of categorisation, and to create a synthesized description for each of the themes, which correspond to the competencies and their definition within the global framework. This work has since been refined by iterative expert review. In particular, it was presented at a stakeholders’ meeting convened by TDR: this was the occasion for external clinical research experts to provide feedback and contribute to the current framework and tools.
Please let us know what you think and get in touch to contribute to future development of this resource!
-
How to proceed from here